Inhibitory PD-1 axis maintains high-avidity stem-like CD8+ T cells
Mice
CD45.2 (C57BL/6J) and B6.GFP (C57BL/6-Tg(UBC-GFP)30Scha/J) mice were purchased from the Jackson Laboratory (strains 000664 and 004354, respectively); CD45.1 (B6.SJL Ptprca), OT-I.CD45.1 (B6(Ly5.1)-(Tg)TCR OT-I-(KO)RAG1) and OT-I.CD45.2 (C57BL/6NAi-(Tg)TCR OT-I-(KO)RAG1) were obtained from the NIAID-Taconic exchange programme (strains 8478, 300 and 175, respectively). XCR1-DTR (B6.Cg-Xcr1tm2(HBEGF/Venus)Ksho) and XCR1-venus (B6.Cg-Xcr1tm1Ksho) transgenic mice15 were gifts from T. Kaisho. OT-I.GFP mice were cross-bred from OT-I.CD45.2 and B6.GFP mice and maintained as a homozygous strain in our laboratory. The majority of mice used were female and aged 6–16 weeks at the beginning of experiments, with a small number of experiments performed in male mice. No significant difference was observed between sex.
Age-matched littermate mice were used to control for litter, cage and age effects. For imaging experiments, two to three mice were used per group in each experiment, and for flow cytometry experiments, three to six mice were used per group. Mice were randomly assigned for experimental groups, and animal experiments were not blinded as the procedures were performed by the same investigator. All mice were bred and maintained under specific-pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care (AAALAC)-accredited animal facility within NIAID and were used (including compliance with tumour size limit) under study protocol LISB-4E approved by NIAID Animal Care and Use Committee (NIH).
Tumour cell line generation
The KP-OVA tumour line was generated by lentiviral transduction of KP6-1B11 cells (a single colony subcloned from the KP1233 cell line derived from a KrasG12D/+Trp53−/− mouse14) with a lentivirus expressing full-length OVA (LRG-EFS-ZsGreen-P2A-OVA). pcDNA3-OVA (Addgene, 64599) was cloned into the lentiviral vector LRG-EFS-ZsGreen-P2A, a plasmid modified from LRG vector61, a gift from J. Shi, and transfected into HEK293T cells, a gift from T. Jacks, for lentiviral production. ZsGreen+ KP6-1B11 cells were sorted into 96-well plates containing single cells per well. Single-cell-derived clones with homogenous morphology and ZsGreen expression were selected and expanded for western blot and flow cytometry validation using anti-OVA (Invitrogen, PA1-196) and anti-mouse SIINFEKL–H-2Kb (25-D1.16) antibodies.
The KP-OVA line was cultured and passaged in RPMI medium containing 10% FCS, l-glutamine (2 mM), penicillin (100 U ml−1), streptomycin (0.1 mg ml−1), sodium pyruvate (1 mM), HEPES (10 mM) and 2-mercaptoethanol (1 mM) at 37 °C under 6.5% CO2.
The MC38-OVA tumour line was generated by lentiviral transfection of MC38 cells with lentivirus expressing full-length OVA (pLV-EF1a-OVA-puro). The MC38 cell line was generously provided by M. Meier-Schellersheim (NIAID, NIH). pcDNA3-OVA (Addgene, 64599) was cloned into the pLV-EF1a-puro lentiviral vector. Transfection was performed on HEK293T cells (Takara, 632180) for lentivirus production. Infected cells were selected with puromycin (8 µg ml−1) and OVA expression was assessed by flow cytometry using anti-mouse SIINFEKL–H-2Kb (BioLegend, 25-D1.16). The MC38-OVA line was cultured and passaged in DMEM medium containing 10% FCS, l-glutamine (2 mM), penicillin (100 U ml−1), streptomycin (0.1 mg ml−1), sodium pyruvate (1 mM) and HEPES (10 mM) at 37 °C under 6.5% CO2. All HEK293T lines were tested negative for mycoplasma, but the tumour cell lines were not tested for mycoplasma.
Tumour induction and protein immunization
For tumour induction, mice were anaesthetized with isoflurane inhalation (2% induction, 1–1.5% maintenance). Mice were shaved on the left flank with a Wahl clipper (Kent Scientific), depilated using Nair hair removal cream and the shaved flank was washed thoroughly with water-soaked gauze. Tumour cells (KP-OVA and MC38-OVA) were collected by washing with PBS, incubated with 0.25% trypsin/EDTA (Thermo Fisher Scientific) at 37 °C for 5 min, then washed with prewarmed RPMI supplemented with 10% FCS. Then, 4 × 105 KP-OVA or 5 × 106 MC38-OVA cells were suspended in 20 µl Hanks’ balanced saline solution (HBSS) and intradermally injected into the left hind flank skin using a 30 G needle. Tumour volumes were measured using digital callipers and the volume was estimated using the formula: volume = ((width2 × length)/2).
For OVA protein immunization, 25 µg ovalbumin (OVA EndoFit, Invivogen) and 12.5 µg poly(I:C) HMW (Invivogen) were reconstituted in 20 µl PBS and were injected intradermally as above, or subcutaneously in the left footpad.
Isolation of CD8+ T cells and adoptive cell transfer
Naive OT-I or wild-type T cells (B6.GFP, CD45.1 or CD45.2) were isolated from spleens and lymph nodes of donor mice with magnetic cell separation (MACS) using mouse CD8a+ T cell isolation kits (Miltenyi Biotec), according to the manufacturer’s instructions. Between 500 and 2 × 103 cells were injected intravenously in 200 µl HBSS into recipient mice at least 1 day before tumour induction or immunization. For CellTrace Violet labelling, CellTrace Violet dye (Thermo Fisher Scientific) was added at a final concentration of 5 µM to 1 × 107 cells per ml suspended in 0.1% BSA-containing PBS and incubated at 37 °C for 10 min, before addition of RPMI with 10% FCS to 10× staining volume and incubated further at 37 °C for 5 min. Cells were washed in RPMI before resuspension in HBSS for adoptive transfer.
In vivo antibody treatment
For blockade of PD-L1 and PD-L2, mice were intraperitoneally injected with 250 µg anti-mouse PD-L1 (10F.9G2, BioXCell) and anti-mouse PD-L2 (TY25, BioXCell) every 2 days starting on day 4 or day 10 after tumour induction. For blockade of PD-1, 250 µg anti-mouse PD-1 (29F.1A12, BioXCell) was injected intraperitoneally into tumour-bearing mice every 3 days starting on day 4 after tumour induction. Rat IgG2a (2A3, BioXCell) and rat IgG2b (LTF-2, BioXCell) isotype antibodies were injected at a dose of 250 µg each as control treatment. In some experiments, PBS was injected intraperitoneally into control animals. No phenotypic differences were observed between IgG-treated and PBS-treated controls.
For depletion of CD4+ T cells, mice were intraperitoneally injected with 100 µg anti-mouse CD4 monoclonal antibody (GK1.5, BioXCell) 3 days and 1 day before tumour induction.
XCR1–DTR depletion
For diphtheria toxin depletion of cDC1 in XCR1–DTR mice, 1 µg diphtheria toxin (Millipore Sigma, 322326) diluted in PBS was injected intraperitoneally into tumour-bearing mice on days 5, 6 and 8 after tumour induction.
Tissue preparation for 3D imaging
Mice were euthanized with sodium pentobarbital through intraperitoneal injection, immediately followed by cardiac perfusion with 10 ml of 1% paraformaldehyde (PFA) prepared from 16% aqueous stock (Electron Microscopy Sciences). Tissues were then collected and fixed for 24 h at 4 °C on a slow shaker in 1 ml fixative buffer (BD Cytofix/Cytoperm diluted 1:4 in PBS; BD Biosciences). Fixed tissues were then washed in 2 ml PBS solution overnight and embedded in 4% UltraPure low-melting-point agarose (Thermo Fisher Scientific) prepared with PBS (cooled to and maintained at 40 °C in a water bath after boiling). Embedded tissues were allowed to solidify on ice. Then, 300 µm agarose-embedded tissue slices were cut using a VT1200S vibrating blade microtome (Leica) at a speed of 0.06 mm s−1 and amplitude of 1.50. Tissue slices were collected into PBS-filled wells in 24-well plates and stored at 4 °C.
Antibody conjugation
Custom antibody fluorophore labelling was performed when specific antibody–fluorophore pairs were not commercially available. Purified antibodies were concentrated using Amicon Ultra 50k MWCO centrifugal filters (Millipore). NHS ester-dye solution (10 mM) was prepared by dissolving NHS ester-dye with DMSO (Sigma-Aldrich). The NHS ester-dye solution and concentrated antibody solution were combined at a 1:9 ratio to yield a final NHS ester-dye concentration of 1 mM, and the solution was allowed to react on ice for 60 min. The reaction mixture was then diluted and concentrated in the centrifugal filters at least three times with PBS at 14,000g centrifugation for 5 min to remove unbound dye. The final antibody–fluorophore conjugates were diluted in PBS and stored at 4 °C. Antibody concentration and degree-of-labelling (DOL) were determined with Nanodrop.
Immunostaining and Ce3D clearing of tissue slices
Tissue slices were incubated in mouse BD Fc Block (1:100; BD Biosciences) prepared in 500 µl BD perm/wash buffer (1:10 diluted in distilled H2O; BD Biosciences) for 24 h at room temperature. For primary antibody staining, blocking buffer was replaced with antibody cocktail prepared in 400 µl perm/wash buffer containing a mixture of fluorophore-conjugated antibodies (1:50 to 1:100 dilution) and incubated for 72 h at room temperature on a slow shaker (60–70 rpm). Stained tissues were then washed in 2 ml perm/wash buffer for another 24 h. After the wash step, post-fixation of the tissue was performed by first replacing the perm/wash buffer with 2 ml PBS for 30 min, followed by post-fixing in 500 µl 1% PFA for 15 min at room temperature, and the samples were then washed again with 2 ml PBS for 30 min. Post-fixed tissues were then transferred into Ce3D solution (see below) for clearing.
All steps were performed in the dark in 24-well plates covered in aluminium foil to protect tissues and fluorophores from light exposure.
For samples with secondary antibody staining, following the wash step after primary staining, the perm/wash buffer was replaced with 500 µl perm/wash buffer containing fluorophore-conjugated secondary antibodies (1:500 dilution) and incubated for another 48 h, washed for 24 h and then post-fixed as described above. A list of the antibodies used is provided in Supplementary Table 1.
For tissue clearing, Ce3D tissue clearing solution was prepared as described previously62 with modifications. In brief, a 10 ml clearing solution was prepared using 5.5 ml 40% (v/v) N-methylacetamine (diluted with PBS; Sigma-Aldrich) and 10 g Histodenz (Sigma-Aldrich) without Triton X-100 detergent. The tube containing Ce3D solution was then incubated in a heated shaker at 37 °C and 150 rpm for at least 1 h until the solid powder was fully dissolved. The prepared Ce3D solution was then stored on a slow shaker at room temperature, wrapped in aluminium foil to protect from light exposure. The refractive index of Ce3D was measured using a digital refractometer, with an expected value of about 1.52.
For tissue clearing, small chambers containing around 700 µl Ce3D solution were prepared on a glass slide (SuperFrost Plus, VWR) by stacking two CoverWell incubation chambers (0.5 mm depth with a 13 mm chamber diameter; Grace Biolabs) on top of an adhesive SecureSeal imaging spacer (Grace Biolabs) to prevent leakage between the silicone chamber and the glass slide. Post-fixed tissue slices were transferred into Ce3D-filled chambers and sealed with a glass coverslip. Tissues were cleared for at least 48–72 h on a gentle shaker (60–70 rpm) at room temperature before imaging.
Before imaging, a shallow imaging chamber was created by stacking two layers of adhesive SecureSeal imaging spacers (2 × 0.13 mm depth) on a SuperFrost Plus glass slide. Up to 4 lymph node tissue slices were placed into an imaging chamber of 20 mm × 20 mm (spacers of 20 mm circular chamber diameter trimmed to a square using a scalpel blade), arranged in a 2 × 2 grid, for batch imaging of tissues from the same experiment. The shallow chamber was then filled with Ce3D solution and gently sealed with a no. 1.5 glass coverslip (VWR).
Laser-scanning confocal microscopy
Volumetric images were acquired using an inverted Leica Stellaris or upright TCS SP8 X confocal microscopes (Leica) equipped with a pulsed white-light laser and four tuneable spectral hybrid detectors. Images were acquired with a ×20 multi-immersion objective (with correction collar adjusted to oil immersion), numerical aperture (NA) = 0.75 and a working distance of 0.66 mm, and were captured at a digital zoom of 1.5 (0.361 µm xy pixel resolution) and 2 µm z step over the full thickness of the tissue slice. Excitation of CellTrace Violet and eFluor 450 was performed using a fixed 405 nm laser line. Twelve- and 16-bit images were typically acquired, although some experimental datasets were acquired as 8-bit images. Image tiles were taken with 5% overlap and merged using the Leica LAS X Navigator application.
For high-resolution imaging of protein co-localization, a digital zoom factor of 2.0–2.5 (0.227–0.284 µm xy pixel resolution) and 1.5 µm z step were used. For anti-NFAT1 stained tissues, images were acquired with a digital zoom factor of 2.0 (0.284 µm xy pixel resolution) with 2 µm z step to provide sufficient lateral resolution for distinguishing cytoplasmic and nuclear NFAT localization.
Chemical bleaching of fluorophores
For imaging experiments involving IBEX iterative staining63, imaged cleared tissue slices were first returned to wells filled with PBS for removal of clearing reagent until the tissue appearance became opaque. Tissues were then transferred into 2 ml perm/wash buffer and washed for 24 h at room temperature, then transferred into new perm/wash buffer-containing wells for two subsequent washes (24 h each) to minimize retention of clearing reagent within the tissues.
To chemically bleach fluorophores with lithium borohydride (LiBH4, STREM Chemicals, 93-0397), bleaching solution was prepared at 1 mg ml−1 LiBH4 concentration in distilled water, as described previously63. Tissue slices were then washed in PBS for 30 min at room temperature, replaced with LiBH4 solution for 45 min and placed ~30 cm from a fluorescent light source. Bleached tissues were then washed again in PBS for 30 min, and finally transferred into a new well containing antibody cocktail solution for subsequent staining steps.
Image analysis
Image preprocessing, segmentation and histocytometry quantification of single cells
Detailed steps of the image processing and analysis pipeline are provided in the Supplementary Methods. In brief, a Python-based computational pipeline was developed and optimized to enable distributed processing of large volumetric datasets on the NIH HPC Biowulf cluster. Raw image data were preprocessed to compensate for spectral spillover and correction for intensity attenuation along the z axis.
Single-cell segmentation was performed with a modified version of Stardist3D64 on nuclei or with Cellpose65 on membrane markers. A small image region was cropped from a representative image and manually annotated as a training dataset, and the custom trained model was used for segmentation of all image datasets from the same experiment. In most cases, Ki-67 nuclear stains were used as the segmentation channel. The nuclear masks were then morphologically dilated to encompass the membrane/cytoplasmic region of the cells, and subtraction of both masks generated a new membrane/cytoplasmic mask. The mean intensity for each channel was determined by summing the masked voxel intensities divided by the sum of all mask voxels for every cell. Output arrays containing both cell coordinates (x, y, z) and mean marker intensities were exported for downstream analyses.
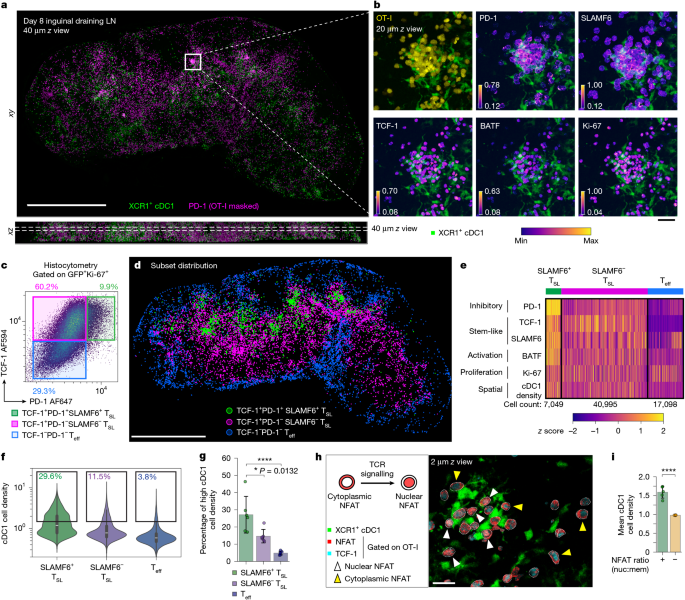
For histocytometry gating of single cells, Python-based scripts were used to visualize marker intensities as two-dimensional histocytometry plots and for gating on further subsets. In brief, scatter plots showing the distribution of mean intensities of protein markers were visualized using the mpl_scatter_density package, and polygonal-shaped gates were drawn using the Polygon class objects from the package shapely, specifying the vertex coordinates of the polygons. The contains method from the Polygon class was used to filter the cells contained within the gates for downstream analysis and visualization. Donor OT-I cells were selected on the basis of Ki-67 and GFP/CD45.1 expression. For gating polyclonal activated CD8+ T cells, Ki-67 and CD8b expression was used. Further subsets were generated based on TCF-1 and PD-1 expression. The spatial distribution of each subset was then visualized using Imaris v.10.0 (Bitplane).
Quantification of marker expression for each subset was visualized with violin plots using the Python-based seaborn visualization library. Principal component analysis was performed using the Python-based scikit-learn library on z-score-normalized marker intensity and plotted using matplotlib library to visualize relative marker expression.
For quantification of TCF-1 expression level among OT-I cells in close proximity to dense cDC1 region, the threshold for the cDC1-densityhigh gate was determined using the mean + 1 s.d. of cDC1-cell density normalized to the maximum cDC1 cell density value of the OT-I cell population (see the ‘cDC1 cell density’ section). OT-I cells above the cDC1-dense threshold (mean + 1 s.d.) were then gated (cDC1-densityhigh) and their relative TCF-1 expression values (normalized to naive T cell expression) were displayed as violin plots. Naive TCF-1 expression was determined by selecting a small patch (about 512 × 512) of the lymph node T cell zone densely populated with naive T cells expressing a high level of TCF-1 and calculating the mean intensity of the TCF-1 channel. Further gating on the TCF-1high subset was performed by selecting cells with a TCF-1 value (normalized to naive T cells) of >0.7.
cDC1 cell density
For determining spatial proximity to dense cDC1 region, a Gaussian smoothing filter of bandwidth σ = 3.6 µm (10 pixels) was applied to the XCR1 channel for each z slice. The mean intensity obtained from a cell’s nuclear mask yields the spatial density of cDC1 at the cell’s centroid.
NFAT quantification
Single-cell segmentation as well as nuclear and membrane/cytoplasmic masks were generated as described in the ‘Image preprocessing, segmentation and histocytometry quantification of single cells’ section. The middle z slice of each cell (the largest cross-section in a 3D cell volume) was used to calculate the ratio between the means of nuclear and membrane/cytoplasmic NFAT intensity. Low-NFAT-expressing cells could lead to false positives and were gated out from analysis. Quantification of the mean cDC1 cell density (normalized) as well as the proportion of cells above the cDC1-dense threshold (determined at mean = 1.0 of the normalized cDC1 cell density described above) was then performed on the remaining NFAT ratio+ and NFAT ratio− subsets.
Kernel density estimation
3D image coordinates of cells of interest were converted to world coordinates by multiplying their voxel dimensions. Kernel density estimation was then performed using the TreeKDE module from KDEpy library, with an isotropic Gaussian kernel (bandwidth σ = 6 µm) across a grid system of 10 µm interval in each axis. Weights were set to normalized protein marker expression (for example, PD-1, SLAMF6) of each cell. To generate a kernel density map for visualization, the density values were summed over a selected z-axis range comprising 80 µm thickness (out of ~300 µm full volume thickness) to reduce clutter. A contour map was then generated using the contourf function in matplotlib library set to a perceptually uniform colourmap for visualization of the probability density on a two-dimensional plot.
Heat-map visualization
Each parameter (protein expression, spatial density) was first standardized to obtain a z score for each cell. The cell population was then subdivided and sorted based on the manual gating strategy defining SLAMF6+ TSL, SLAMF6− TSL and TCF-1− Teff cells. A perceptually uniform colourmap was applied to display the relative z score of each parameter. Note that both screen and print resolutions are not sufficient to enable discrimination of single cells within the heat map, which contains the total population of OT-I recovered from the lymph node tissue slice (>65,000 cells in the dataset shown in Fig. 1e).
Visualization
For visualization of protein markers expressed by gated cells, segmented labels (nuclear masks) of the gated cells were processed with the morphological dilation tool (radius = 6) from scikit-image library to generate a new mask encompassing the cytoplasmic/membrane region of the cells. Individual image channels were then multiplied with the mask to generate a new image displaying only protein marker intensities masked within the cells of interest. These masked images were then visualized using Imaris v.10.0 (Bitplane). To visualize the relative protein expression level, perceptually uniform colourmaps were used and the min/max scaling (contrast and brightness) of each channel was individually adjusted to avoid under- and over-saturation of the marker intensity.
Animation
For animation of 3D imaging datasets, the napari-animation library was used and an animation script based on the instructions provided by the developers was made to generate keyframes specifying the camera positions and angles, image layer’s colourmap, adjustments of contrast/brightness, as well as clipping planes for animating transition between layers and for focusing on thin cross-sections of the imaging volume. The output video file was further processed and edited in Davinci Resolve v.18.6 (Blackmagic Design) to include annotations with text and graphic items.
Tissue preparation for flow cytometry
Lymphocytes were isolated from lymph nodes and spleens and made into single-cell suspensions using a syringe plunger and 100 µm or 70 µm cell strainers (MACS SmartStrainer, Miltenyi Biotec). Around 3 × 106 cells were used in subsequent staining steps for flow cytometry analysis. Dendritic cell isolation was performed as described previously66. In brief, lymphoid tissues were sliced into small fragments using a scalpel blade and incubated in a digestion mix of collagenase type III (Worthington, 1 mg ml−1) and DNase I (20 μg ml−1) and vigorously mixed for 25 min at room temperature, followed by addition of 0.1 M EDTA solution at 1/10 digestion volume for 5 min to dissociate lymphocytes from dendritic cells. Tissue debris was filtered out by passing the cell suspension through a 70 µm nylon mesh.
T cells from skin tumour samples were isolated as described previously67. In brief, a 1 cm × 1 cm tumour-containing skin patch was collected into collagenase type III (Worthington, 3 mg ml−1), finely chopped with scissors and incubated at 37 °C for 90 min before pressing through 70 µm cell strainers. For spleen and tumour samples, cells were also treated with red blood cell lysis buffer before staining. Cell counts in lymph node and spleen were determined using an automated Cellometer T4 cell counter (Nexcelom Bioscience).
Flow cytometry
For detection of polyclonal OVA-specific CD8+ T cells, cells were first incubated with phycoerythrin-conjugated H-2Kb–SIINFEKL tetramer (1:100, NIH Tetramer Core) for 20 min at 37 °C, washed, followed by cell surface marker staining for 25 min at 4 °C. Mouse BD Fc Block (1:200, BD Biosciences) was also included during the cell surface marker staining step. A fixable LIVE/DEAD near-infrared staining dye was used for determining cell viability. For detection of intracellular proteins, stained cells were further treated with fixative and stained for antibodies against intracellular proteins using FOXP3/transcription factor staining buffer kit according to the manufacturer’s protocol (eBioscience). For tumour samples, CountBright Plus Absolute beads (Thermo Fisher Scientific) were added before sample acquisition. The samples were acquired using the BD Fortessa (BD Biosciences) system and analysed using Flowjo 10 (TreeStar). A list of the antibodies used is provided in Supplementary Table 1.
Imputed affinity index
Tetramer binding is an estimation of TCR avidity (based on the multivalent binding strength of the TCR–pMHC complex), which in itself is a function of TCR affinity (monovalent TCR–pMHC binding), the amount/density of expressed TCRs, as well as the presence of co-receptors (such as CD8) that can potentially influence binding ability in a solution-based measurement.
To estimate the TCR affinity of tetramer-stained cells, an imputed affinity index was derived by dividing the tetramer staining intensity by the TCR (CD3) staining intensity (tetramer/CD3 ratio) of the single cells. This is possible because tetramers elute from labelled T cells in the wash buffer during incubation steps in reasonable proportion to affinity, in particular the off-rate of the interaction26,68,69,70,71. When normalized to surface TCR expression to account for avidity differences among T cells, we can use the measured staining as a proxy for this off-rate and, therefore, affinity. Cells were post-fixed after the surface marker staining step, before intracellular antibody staining, to minimize continued elution of tetramers over time.
Further controls for secondary factors affecting this off-rate such as CD8 co-receptor binding, as well as TCR level/density, were performed through direct comparison with monoclonal OT-I TCR transgenic cells under control and checkpoint treatment conditions as described in the main text.
Given that tetramer binding varies from batch to batch and is more sensitive to incubation conditions compared with antibody staining, a z-score for each subset from each animal was derived from the log-affinity index (log(tetramer/CD3 ratio)) of the tetramer-stained cells, standardized with reference to SLAMF6+ TSL cells of the control IgG-treated group pooled from the same experimental cohort. The mean of the z score, mean z(log(affinity index)), therefore estimates the distribution of TCR affinity of each subset and treatment group in relation to SLAMF6+ TSL cells of the control IgG-treated group.
A normalized ratio was also derived from the imputed TCR affinity indices of tetramer-stained cells, by normalizing the affinity indices of each subset from each animal to the reference affinity index of SLAMF6+ TSL cells from control IgG-treated group pooled from the same experimental cohort. This normalized ratio, mean affinity index, estimates the relative TCR binding affinity of each subset and treatment group in relation to SLAMF6+ TSL cells of the control IgG-treated group. When specified, other reference subsets (for example, day 6 TCF-1+ TSL cells) were also used for normalization.
To plot the mean CD8a expression versus log(affinity index) of OT-I and polyclonal T cells in Extended Data Fig. 7j, the mean CD8a expression values and the mean imputed log-affinity indices were obtained from OVA–tetramer+SLAMF6+ TSL cells of each sample and normalized to the reference mean CD8a value and mean log-affinity index, respectively, of all OVA–tetramer+SLAMF6+ TSL cells pooled from control IgG-treated animals from the same experimental cohort.
As the mean affinity indices of SLAMF6+ TSL cells in some animals in the anti-PD-L1/2-treated group were very low, which led to negative log-transformed values, the imputed affinity indices of all OVA–tetramer+ cells from the same experimental cohort (pooled from both control IgG-treated and anti-PD-L1/2-treated samples) were first translated with the formula: affinity index = affinity index + (1 − min(affinity indexpooled)) to ensure that log-transformed imputed affinity indices remain positive and to allow for normalization with the control IgG-treated SLAMF6+ TSL cells.
Modelling of TCR affinity and cell states
In the course of these studies, we observed a number of outlier responses among the treated individual mice. Such results are not unexpected based on the known variation in TCR repertoire between inbred animals13. A linear modelling analysis was conducted to determine whether these outlier events affected the conclusions of our flow cytometry studies.
The TCR affinity of single cells was quantified as the log-ratio of tetramer staining to CD3 staining (log(tetramer/CD3 ratio)). Within each experiment, these log-ratios were standardized with reference to the control IgG-treated SLAMF6+ TSL cells, to emphasize the differences in affinity between cell states and treatment groups. The three major subsets of OVA–tetramer+CD8+ T cells: SLAMF6+TCF-1+ TSL, SLAMF6−TCF-1+ TSL and TCF-1− Teff cells were defined as distinct cell states. Standardization was performed separately for each experiment, to control for experiment-to-experiment staining variability, because affinity measurements differed substantially across experiments. Cellular affinity was then regressed on cell state, treatment and their interaction using a Bayesian multilevel model with a Gaussian likelihood. Hyperparameters for the effect of mouse identity on the intercept and on the cell state slopes were included to account for mouse-to-mouse variability.
Predictions from this model were simulated without mouse-to-mouse variability to isolate the effects of cell state and treatment on the expected distributions of cellular affinity.
In a second model, the number of cells in each cell state from each animal was log-transformed. These log-counts were then standardized across all groups of cells. Cell counts were regressed on cell state, treatment and their interaction using a Bayesian multilevel model with a Gaussian likelihood. Hyperparameters for the effect of mouse identity on the intercept and on cell state slopes were included to account for mouse-to-mouse variability. Predictions from this model were simulated without mouse-to-mouse variability to isolate the effects of cell state and treatment on the expected cell counts.
The simulated predictions from the first model provided expected distributions of log-affinity relative to the mean of control IgG-treated SLAMF6+ TSL cells for each combination of cell state and treatment. The fraction of cells with log-affinity greater than the mean of control IgG-treated SLAMF6+ TSL cells was simply the fraction of cells above 0 in each distribution. These fractions were multiplied by the expected total number of cells for each combination of cell state and treatment, provided by the simulated predictions of the second model. This gave the expected number of cells greater than the mean log-affinity of control IgG-treated SLAMF6+ TSL cells (above average TCR affinity threshold) for each combination of cell state and treatment.
Statistics
Statistical tests were performed in Prism 9.0 software (GraphPad). Data analyses were performed using unpaired two-tailed Student’s t-tests or one-way ANOVA with Tukey’s post hoc multiple comparison test, as specified in the text or figure legends. P < 0.05 was considered to be significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
نشر لأول مرة على: www.nature.com
تاريخ النشر: 2025-11-26 02:00:00
الكاتب: Jyh Liang Hor
تنويه من موقع “yalebnan.org”:
تم جلب هذا المحتوى بشكل آلي من المصدر:
www.nature.com
بتاريخ: 2025-11-26 02:00:00.
الآراء والمعلومات الواردة في هذا المقال لا تعبر بالضرورة عن رأي موقع “yalebnan.org”، والمسؤولية الكاملة تقع على عاتق المصدر الأصلي.
ملاحظة: قد يتم استخدام الترجمة الآلية في بعض الأحيان لتوفير هذا المحتوى.




